
|
|
| Five ways to reduce Legionnaires' disease risk in your facility |
| Legionnaires' disease cases are increasing dramatically, and older adults and healthcare facility patients are at greatest risk. Luckily, 9 out of 10 Legionnaires' disease cases are preventable.1 Here are five simple ways to reduce your outbreak risk. |
|
|
|
1. Validate the effectiveness of your water management plan with routine testing
Healthcare facility water systems can be large, complex, and harbor pathogens.  Maintenance, renovations, and service interruptions can cause hazardous conditions. The Centers for Disease Control and Prevention (CDC) recommends that healthcare facilities that provide inpatient services to people over 50 or with COPD or weakened immune systems consider validating their control measures with routine environmental testing for Legionella.2 Testing is also the best way to ensure your facility meets the Joint Commission hospital accreditation Environment of Care standard to "minimize pathogenic biological agents in cooling towers, domestic hot and cold water systems, and other aerosolizing water systems."3 Maintenance, renovations, and service interruptions can cause hazardous conditions. The Centers for Disease Control and Prevention (CDC) recommends that healthcare facilities that provide inpatient services to people over 50 or with COPD or weakened immune systems consider validating their control measures with routine environmental testing for Legionella.2 Testing is also the best way to ensure your facility meets the Joint Commission hospital accreditation Environment of Care standard to "minimize pathogenic biological agents in cooling towers, domestic hot and cold water systems, and other aerosolizing water systems."3
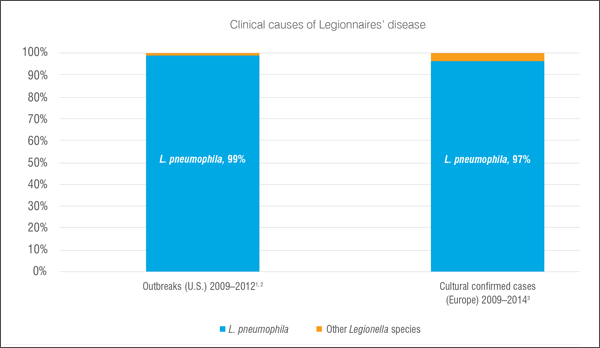
2. Test for Legionella pneumophila, the bacteria that causes 99% of Legionnaires' disease
outbreaks
A single species of Legionella bacteria, Legionella pneumophila  is the primary cause of Legionnaires' disease. This species was responsible for 99% of the outbreaks tracked by the CDC Centers for Disease Control and Prevention.4 is the primary cause of Legionnaires' disease. This species was responsible for 99% of the outbreaks tracked by the CDC Centers for Disease Control and Prevention.4
 Accordingly, Veterans Administration healthcare facilities are required to test for Legionella pneumophila every quarter.5 The World Health Organization advisors suggest testing for Legionella pneumophila, "not Legionella species, since this genus contains many species that do not cause illness."6 The Legiolert* Test, an accurate and highly reproducible new culture based test, opens the way for healthcare facilities to specifically detect and respond to Legionella pneumophila.
Accordingly, Veterans Administration healthcare facilities are required to test for Legionella pneumophila every quarter.5 The World Health Organization advisors suggest testing for Legionella pneumophila, "not Legionella species, since this genus contains many species that do not cause illness."6 The Legiolert* Test, an accurate and highly reproducible new culture based test, opens the way for healthcare facilities to specifically detect and respond to Legionella pneumophila.
3. Rely on a culture test, the gold standard for Legionella testing
Although rapid tests, such as PCR, offer faster results in an outbreak situation, culture tests are the only reliable source of accurate quantification of Legionella. PCR and direct fluorescent antibody (DFA) tests do not discriminate between live Legionella and bacteria that have already been controlled, so are not a threat to patients. Lateral flow antibody "field tests" only detect a single serotype of Legionella pneumophila and have high false-negative results, often missing the pathogen completely. In contrast, the Legiolert Test provides a confirmed culture result for all serogroups of L. pneumophila.
4. Make sure your results are accurate and reproducible
Routine testing for Legionella pneumophila is the best way to ensure that your Water Safety Plan  is effective. is effective.
 In addition to identifying the presence of L. pneumophila in your system, you'll need consistent data about the exact quantity and locations of the bacteria to respond appropriately. The Legiolert Test has been demonstrated to meet or exceed the accuracy of traditional methods for Legionella testing and delivers confirmed results in only seven days, significantly faster than the 10–14 days for traditional culture tests.7 And unlike traditional methods, which require subjective counts of colonies on petri plates, Legiolert results are 99% repeatable and reproducible (ISO/TR 13843 study); you have consistent data upon which to make high stakes decisions on potential water quality issues.
In addition to identifying the presence of L. pneumophila in your system, you'll need consistent data about the exact quantity and locations of the bacteria to respond appropriately. The Legiolert Test has been demonstrated to meet or exceed the accuracy of traditional methods for Legionella testing and delivers confirmed results in only seven days, significantly faster than the 10–14 days for traditional culture tests.7 And unlike traditional methods, which require subjective counts of colonies on petri plates, Legiolert results are 99% repeatable and reproducible (ISO/TR 13843 study); you have consistent data upon which to make high stakes decisions on potential water quality issues.
5. Choose a laboratory that is accredited for Legionella testing, the highest standard for quality
Laboratories that perform Legionella pneumophila testing with a method that is part of their Accreditation and Quality System demonstrate that their testing is performed at the highest level of quality. Accreditation also affords the best legal protection if testing results ever come under question.
ISO 17025 and EMLAP (AIHA) are nationally recognized accreditation programs that include a Legionella field of testing.
Learn more about Legiolert and reducing your Legionnaires' disease risk.
|
|
References:
- Vital Signs, Centers for Disease Control and Prevention (CDC). June 2016;1.
- Developing a Legionella water management program. Centers for Disease Control and Prevention (CDC). 2016;21–27.
- Standard EC.02.05.01 The critical access hospital manages risks associated with its utility system. EP 4. Joint Commission–Standards Revisions Related to Medical Equipment and Utility System Maintenance. 2014.
- Surveillance for waterborne disease outbreaks associated with drinking water–United States, 2011–2012. Morbidity and Mortality Weekly Report (MMWR) 2015;64(31);842–848 and 2009–2010 MMWR. 2013;62(35):714–720.
- Veterans Health Administration, U.S. Department of Veterans Affairs. Prevention of healthcare-associated Legionella disease and scald injury from potable water distribution systems. Washington, DC: Veterans Health Administration; C– 2014. VHA Directive 1061.
- World Health Organization (WHO). Draft–Background paper on water microbiologically safe water and microbiological parameters for Revision of Annex I of the council directive on the quality of water intended for human consumption. 2016:27.
- Sartory DP, Spies K, Lange B, Schneider S, and Langer B. Evaluation of most probable number method for the enumeration of Legionella pneumophila from potable and related water samples. Letters in Applied Microbiology. 2017; 54(4):271–275.
|
|
|
|